(→Design 3: I wonder if too much heat is flowing through your stand to the ground.) |
Beth Ogilvie (talk | contribs) m (Reverted edits by 217.146.123.146 (Talk); changed back to last version by Beth Ogilvie) |
||
| (3 intermediate revisions by 2 users not shown) | |||
Revision as of 18:46, 18 April 2007
Solar Cooker
Preliminary thoughts
After I get my box cooker working well, I want to try a solar cooker for backpacking. What I have in mind is a funnel cooker where the structure is provided by a sleeping pad rolled into a cone and stuck into the open top of a backpack that is cleverly propped up at a good angle (using a rock or perhaps a hiking pole or two). The inside of the funnel would be lined with a piece of space blanket. The pot (my little titanium pot, which is gradually getting blackened by alcohol stove experiments) would sit in an oven bag on a little pad. (Fleece hat? Spare socks?)
Do you think it would work? Got any suggestions before I begin experimenting? The only extra weight (things I wouldn't be carrying anyway) would be the bit of space blanket and the oven bag, probably 1-2 oz, which is good because if the extra weight were more than about 3 oz it probably wouldn't make the cut. If it didn't actually boil water, but got it up to 165 F (without taking all afternoon) that would be satisfactory. So in other words, here are the design goals:
- Bring 2 cups of water to 165 F in an hour
- Add less than 3 oz of extra weight
Do you think the heat would damage a Thermarest pad? Would a closed cell foam pad be better? Would you expect it to work better or worse at 10,000' than at sea level (assuming a bright sunny summer day)?
Thanks. Beth Ogilvie 01:00, 9 March 2007 (UTC)
- It should work, I think. The atmosphere absorbs less of the solar radiant flux at high altitude. Less longwave radiation is radiated down from the atmosphere, too. But I've argued elsewhere that this is a minor matter. The volumetric heat capacity of air is less, so the heat transfer coefficient for convective heat transport from warm surfaces to the air is reduced.
- Neglecting losses and inefficiencies, a 30 inch diameter collector will heat two cups of water 14 °C/minute. (Net radiant flux: 1000 W/m^2; water mass: 0.5 kg; water heat capacity: 4200 J/kg-°C; collecting area: 0.5 m^2).
- Most polymers are not permanently changed by heating to 100°C although their strength is reduced. Closed cell foam is cheaper to replace, if damaged.
- A design that will be robust in the presence of wind loads will be difficult with your weight budget. A 5 m/s wind (11 mph) corresponds to a load of 25 N/m^2 for a drag coefficient of 2. That will be several pounds of force on your collector. Bicycle spokes, tent stakes, cord stays, etc. may have a role.
- Good luck, Walter Siegmund 14:25, 9 March 2007 (UTC)
Design 1

It's hard to see the details of this, but the lightweight inflatable pad is bent into a "U" shape with the aid of velcro in one spot, then the space blanket is attached with a few small metal binder clips. The pot balances precariously on its stand which is on a small aluminum plate. A couple of rocks underneath help keep it upright.
Disadvantages of this design: The space blanket is unruly and the whole thing is easy to tip over.
Design 2
 |
 |
For this design, I cut open a new space blanket sack, which wrapped around the pad in a much more cooperative way, and I used trekking poles, attached with string, to provide structure. It didn't heat very well in the V configuration, though (the sun was shining across the V) so I turned it to face the sun and put one side down as shown in the 2nd photo.
Disadvantages of this design: It's a bit fussy to setup, and if it jumps in the wind at all, the pot tips over. Also it doesn't heat that well, though perhaps moving the pot away from the rear reflector, and tipping the rear reflector forward (this is April in N California) would help with that.
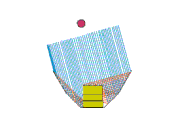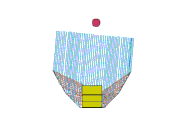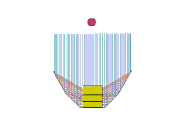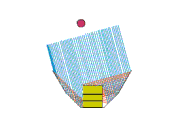
Design 3

This is my favorite design, even though it doesn't heat so well either! But maybe we can fix that... It sure is easy to setup. The pad stands on its side with the space blanket tube around it. The middle segment of a trekking pole holds the "U" open, and one piece of string holds the arms in. Another piece of space blanket sits flat on the ground, and the pot and stand (about 2" high) are in the oven bag.
Advantages of this design: It's easy to setup and the pad can move a little in the wind without tipping the pot over. It doesn't require the extra little plate to stabilize the pot stand.
So how can I make it heat better? There's a lot of collector area, but maybe it's not focused on the pot. Should I try tipping the back and sides so they slant down towards the pot?
Beth Ogilvie 20:09, 16 April 2007 (UTC)

Pardesi Double Funnel Cooker
- I wonder if too much heat is flowing through your stand to the ground. Perhaps you might place the pot/stand on foam. If that helps, the stand may be too conductive. What is the stand made of? Does a magnet stick to it? If so, you might replace it with a stand made of 1.5 mm thick stainless steel wire, or 3 mm diameter thin-wall stainless tubing. Stainless steel is the lowest-conduction, readily-available metal. Titanium is less conductive, but can't be soldered. Stainless-steel can be silver-soldered with hardware store torch, silver-solder and flux.
- Perhaps you can adapt the idea of the double funnel to improve the concentration on your pot. The advantage of this approach is that the surfaces are conical and that makes it easy to analyse, i.e., incident and reflection angles are equal. Walter Siegmund 02:27, 18 April 2007 (UTC)
Solar Water Heater
 |
 |
My goal here is to make an ultralight version of the Sunshower II, which claims to heat 2.5 gallons from 60°F to 108°F in 3 hours. I would be happy with 1 gallon (approx 4L) in 1.5 hours.
My design uses a water bag and a piece of black plastic. After the water is hot, I'll plug in the shower adaptor.
First I tried just the water bag, but with an air space (perhaps 2" at the peak?) above the water (left photo). Then I tried no air space in the bag, but with a turkey-size oven bag wrapped around it (right photo). Either way, it only heated about 15°F/hour.
The Sunshower is blacker at the back than my design, and has a clearer top membrane. I can't do much about the top, but maybe I can make the back blacker. I could wiggle a piece of black plastic inside the bag (and try to flatten it out) so there'd only be one layer of translucent water bag before the black plastic. Would that help? (This is for washing, not eating, so it doesn't matter if plastic leaches into the water.) Also I had the whole thing sitting on a plastic garden table, and maybe it was cooling off underneath. I think I could afford (in my weight budget) a small piece of closed cell foam to put under it - and have it sitting on the cement.
Beth Ogilvie 20:09, 16 April 2007 (UTC)
- You are losing 19 W/m2°C out the bottom (only a single air boundary layer that is wind-forced ventilated). Foam 10 mm thick will reduce this to 2.6 W/m2°C. Adding a layer or two of Thermorest would make loss through the bottom negligible.
- On the sides and top, a single air gap 10 mm or more has a conductivity of 1.7 W/m2°C (including the exterior air boundary layer).
- Leaving an air space in the inner bag above the water will have little effect. Water vapor condensing on the inside of the bag will transfer heat efficiently across that air space.
- You won't gain nearly as much by making the back blacker. Anything that looks dark grey or black is probably 80 or 90% absorbing. You won't increase that by a factor of two! You could get a factor of two or three with one or more reflectors, or by using larger bags. The latter approach will increase the surface area and the heat loss, though.
- Your 4 kg of water has a heat capacity of 4*4200 J/°C or 17 kJ/°C. Assuming 1 kW/m2 and 0.2 m2 of area, you have 200 W. The heat rise with no losses is 200/17,000 = 0.012 °C/s or 40 °C/hr.
- I suggest...
- Support the inner bag on foam, Thermorest, or a 30 mm enclosed air gap (bubblepack, or egg cartons inside outer bag).
- Maintain a 10 mm gap between the inner and outer bags on 5 sides. I'm not sure how you do that if there is wind, but it is important. Bubble pack might work.
- Make the absorber blacker and/or crumble it up, but only if it is very easy.
- Add one or more reflectors.
- The last two should not be necessary. Walter Siegmund 00:54, 17 April 2007 (UTC)
- Ashok Kundapur says "The water heater should work, especially if you put it over a small heap of dry leaves. I had fabricated such low cost water heaters, of 100 and 200 lit capacity, way back in '80ies. But could not get good plastic materials. The freely available ones were supposed to leach out carcinogenic chemicals, and hence had abandoned the project."
- Thanks for all the suggestions! I'll put some insulation under it and try some bits of bubble pack for spacing between the bags. Now how about the cooker designs, especially #Design 3 above? --Beth Ogilvie 21:35, 17 April 2007 (UTC)